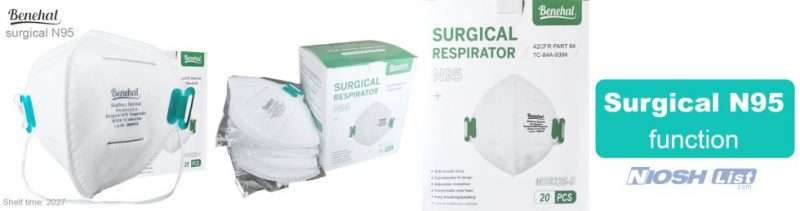
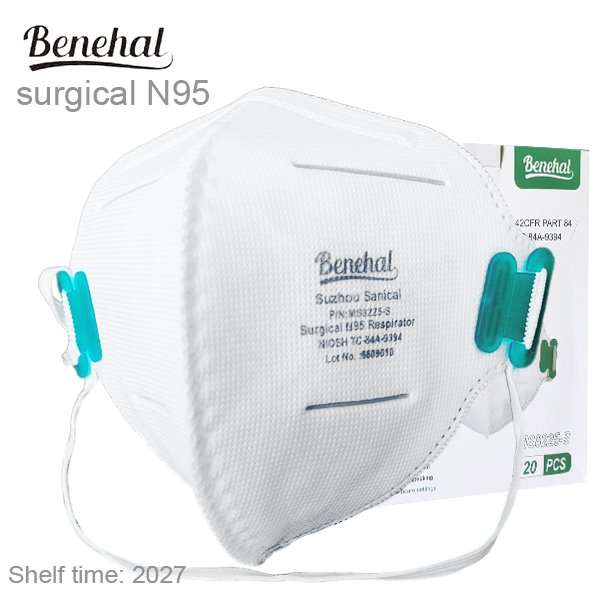
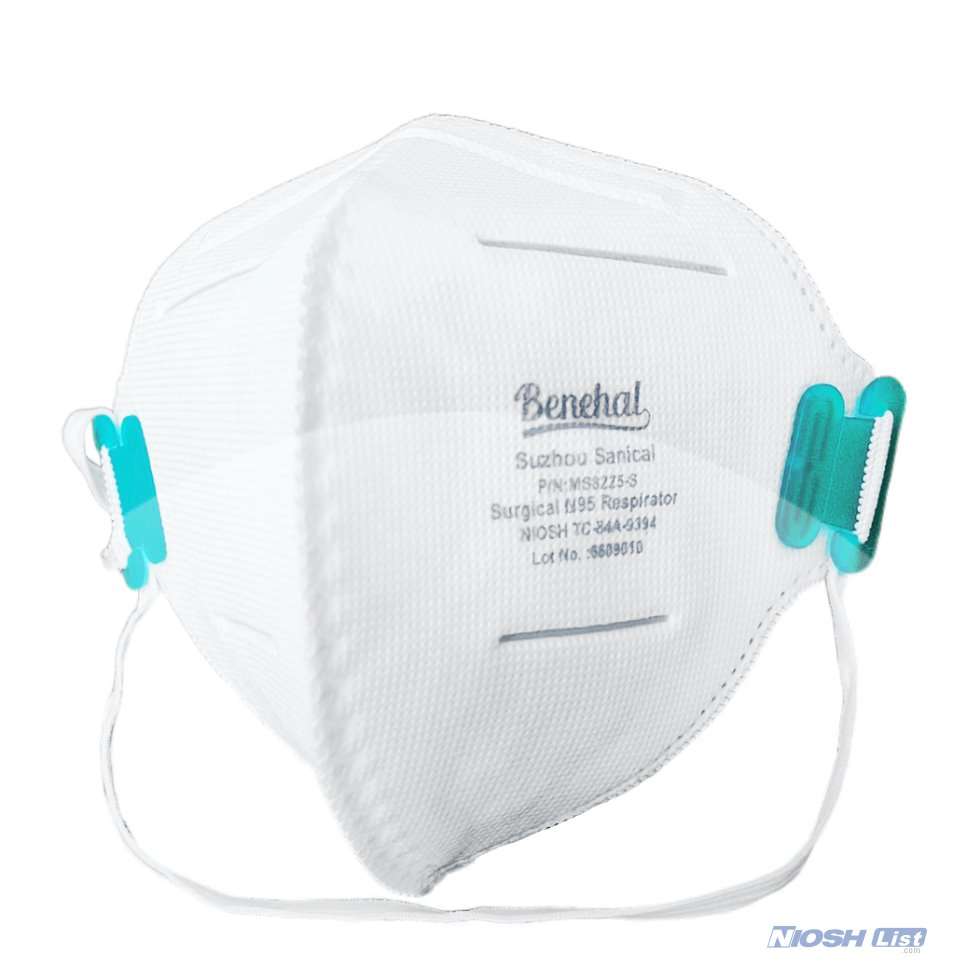
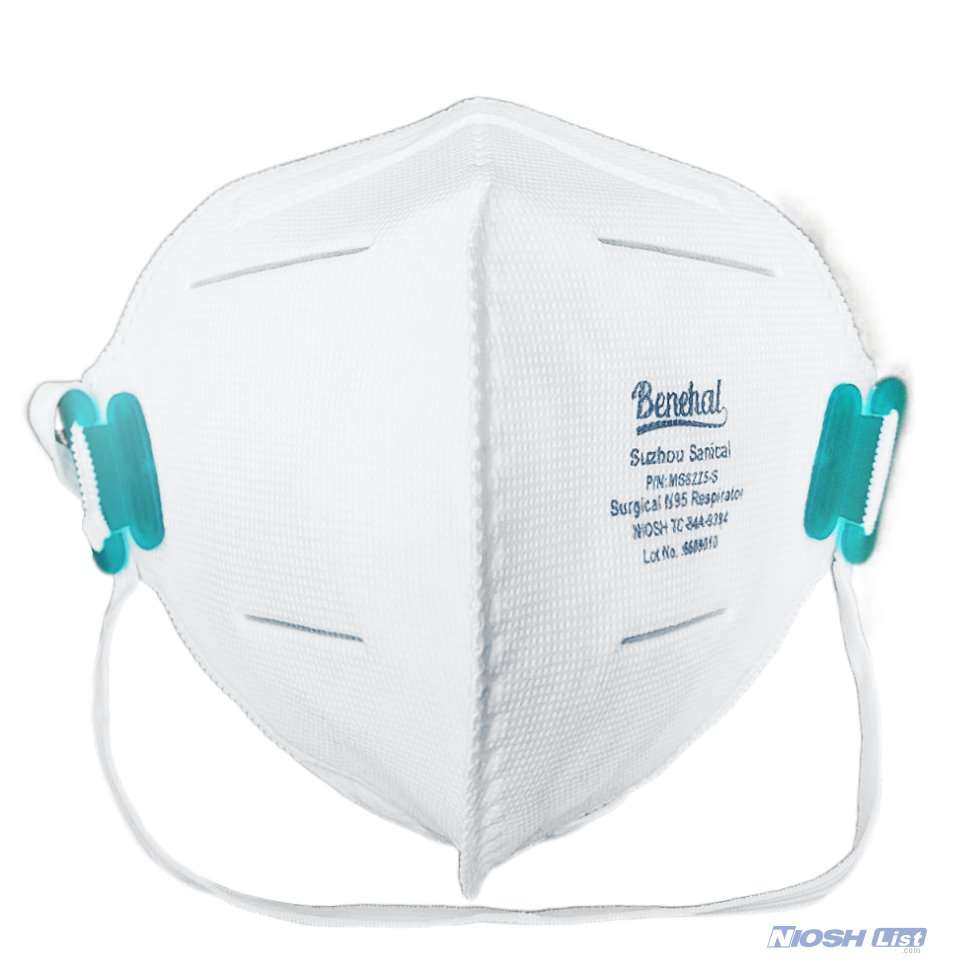
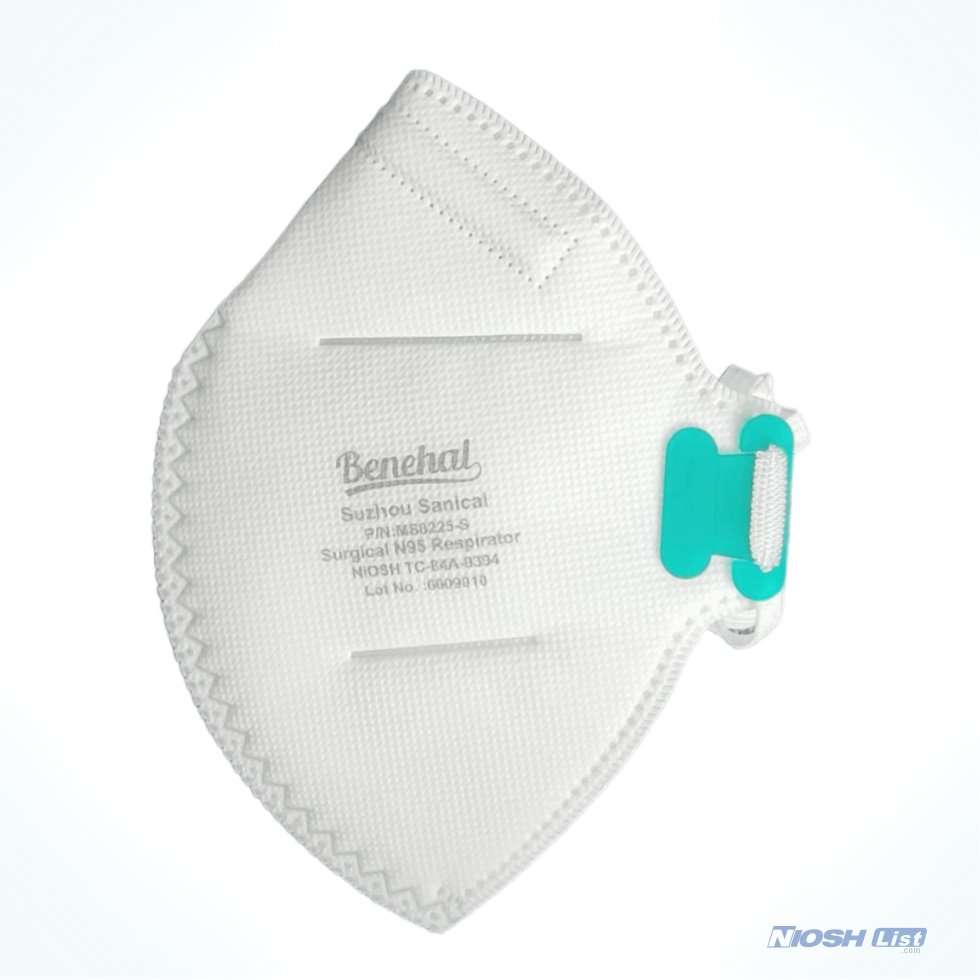
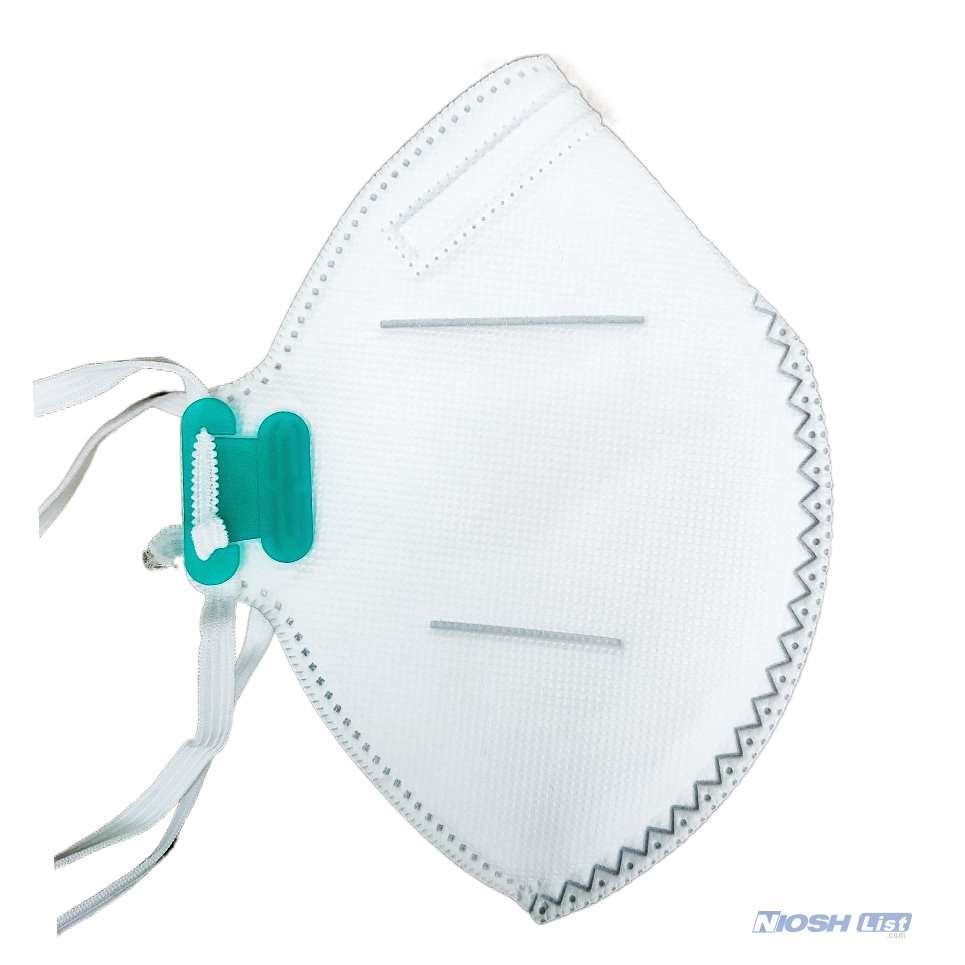
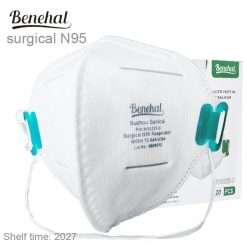
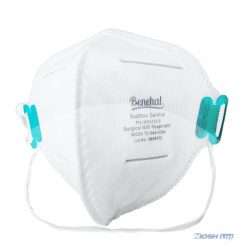
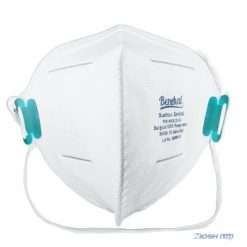
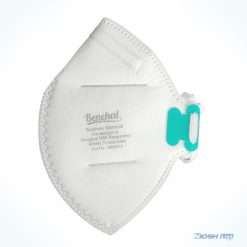
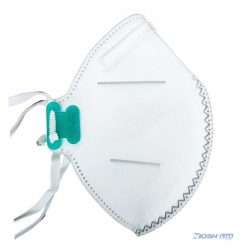
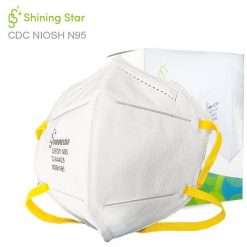
Benehal MS8225-S surgical N95 mask is CDC NIOSH-approved surgical N95 mask. As a surgical mask cleared by FDA, it passed strict testing with more than 95% filtration effeciency. Benehal MS8225-S medical face mask helps provide quality, reliable, and convenient respiratory protection of at least 95 percent filtration efficiency against certain non-oil based. Manufacture Suzhou Sanical Protective Product Manufacturing Co., Ltd. Uses a variety of innovative technologies and features to help you meet your respiratory protection and comfort needs. Instocking LLC is an authorized distributor of Suzhou Sanical. All masks we offered are 100% genuine guarantees.
Potential settings and applications for Benehal MS8225-S folding surgical mask
Healthcare personnel in healthcare settings like hospitals and clinics, operating rooms, TB wards, and laboratories. For the general public: consumer grocery shopping, classrooms, pharmacies, dental clinics, office workplace environments, public transit, airports and airplanes, restaurants, neighborhood walking, and jogging, etc.

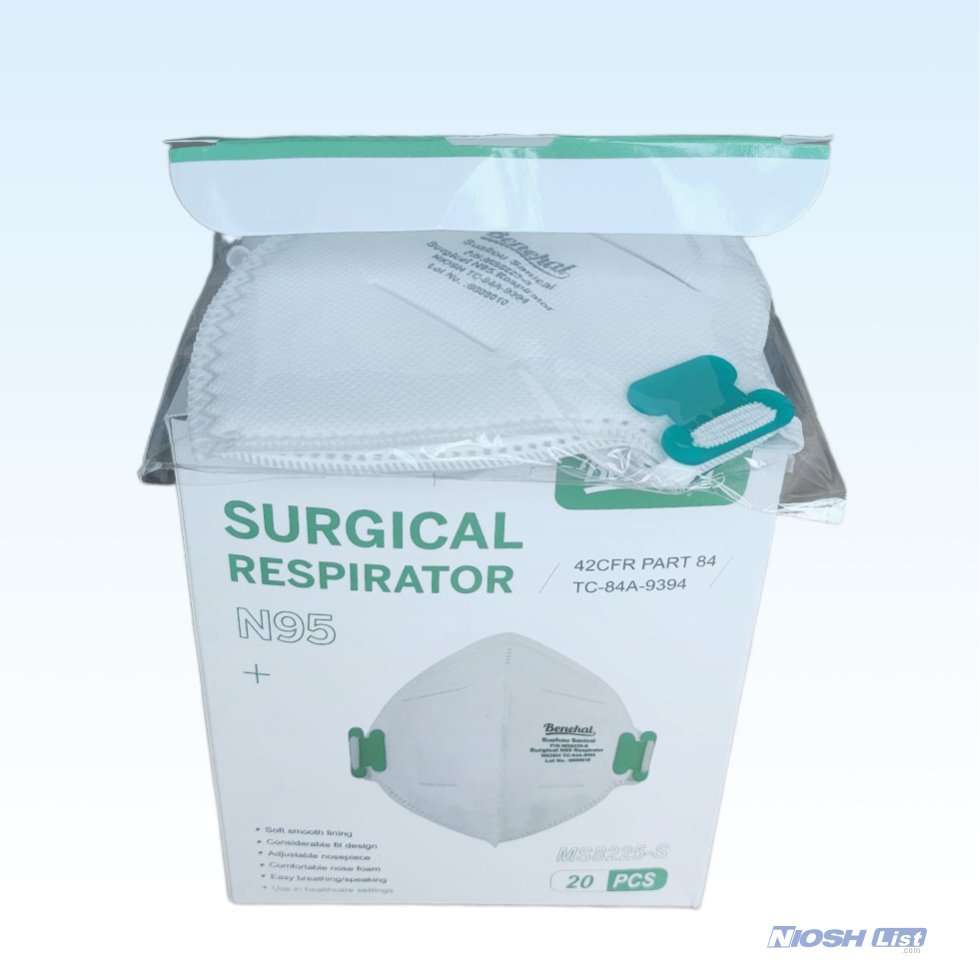
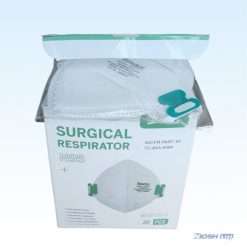
Benehal MS8225-S N95 Mask with Individual Package Overview
- NIOSH approved N95 Respirator- Approval Number: 84A-9394 – certified for at least 95% particulate filtration efficiency.
- Fits most facial sizes with standard American size.
- Elastic headloop and invisible nose clip offer a customized seal.
- Upgraded protection with 4 layers.
- Comfortable to wear with skin-friendly material.
- Each mask comes with individual bags.
- Long shelf life 5 years.
- FDA cleared surgical mask.
- Approved as a NIOSH N95 filtering facepiece respirator for use in healthcare settings.
- Meet surgical mask standards for biocompatibility, flammability, and fluid resistance at 120mmHg.

Sanical Benehal NIOSH N95 Respirator MS8225-S Certification
NIOSH approved with TC number TC-84A-9394
Sanical Benehal NIOSH N95 Respirator MS8225-S Details
Size Medium
20 per box
Shelf life 5 Years
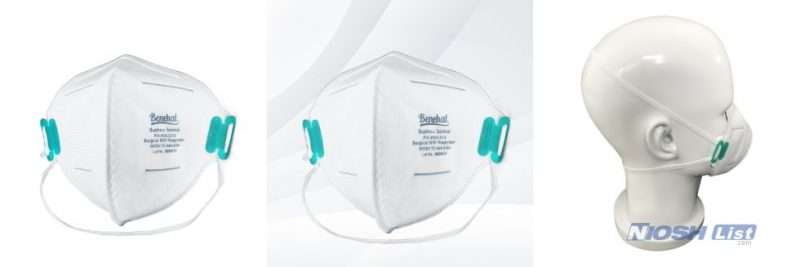
Benehal MS8225-S Surgical Filtering Face Piece Cautions and Limitations
A – Not for use in atmospheres containing less than 19.5% oxygen.
B – Not for use in atmospheres immediately dangerous to life or health.
C – Do not exceed maximum use concentrations established by regulatory standards.
J – Failure to properly use and maintain this product could result in injury or death.
M – All approved respirators shall be selected, fitted, used, and maintained in accordance with MSHA, OSHA and other applicable regulations.
N – Never substitute, modify, add, or omit parts. Use only exact replacement parts in the configuration as specified by the manufacturer.
O – Refer to User’s Instructions, and/or maintenance manuals for information on use and maintenance of these respirators.
S – Special or critical user instructions and/or specific use limitations apply. Refer to the instruction manual before donning.

Benehal MS8225-S Medical Face Mask Use Instruction
1. Failure to follow all instructions and limitations concerning the use of the respirator and/or failure to wear the respirator for the whole duration of exposure to contaminants can seriously reduce the performance characteristics of the respirator and lead to illness, injury or death.
2. Before use, the wearer must first be trained by the employer in the correct use of the respirator in accordance with applicable safety and health standards.
3. OSHA standards including 29CFR1910.134 (e) (5) and CSA standard Z94.4-93 require that the wearer be fit-tested.
4. Discard the respirator and replace with a new one if:
• Excessive clogging of the respirator causes breathing difficulty.
• The respirator becomes damaged.
5. Leave the contaminated area if dizziness, irritation or other distress occurs.
6. This respirator contains no components made from natural rubber latex.
7. This respirator has been tested and small punctures within ultrasonic welding areas are normal and do not interfere with the respirator compliance with Part
84 approval requirements: (A) filtering facepieces are to be inspected prior to each use to assure there are no holes in a breathing zone other than the punctures within ultrasonic welding areas and no damage has occurred, and (B) enlarged holes resulting from ripped or torn filter material within ultrasonic welding areas punctures are considered damage.
S-Special or Critical User Instructions:
This respirator has been approved as a NIOSH N95 filtering facepiece respirator, for use in healthcare settings, as a Surgical N95 Respirator conforming to recognized standards for biocompatibility, flammability, and fluid resistance at 120mmHg.