As a surgical mask, GIKO 1400 is classified as Class II Medical Device under Regulation 21 CFR 878.4040. It is intended to be worn by operating room personnel during surgical procedures to protect both the surgical patient and the operating room personnel from the transfer of microorganisms, body fluids, and particulate material. 510(k) Premarket Notification number for Giko 1400 FDA Approved surgical n95 masks is K063334.

GIKO 1400 N95 NIOSH Respirator Overview
GIKO 1400 is NIOSH approved with approval number TC-84A-4282.
As surgical masks in white color, they are designed to be fluid resistant to splash and spatter of blood and other infectious materials according to ASTM F1862.
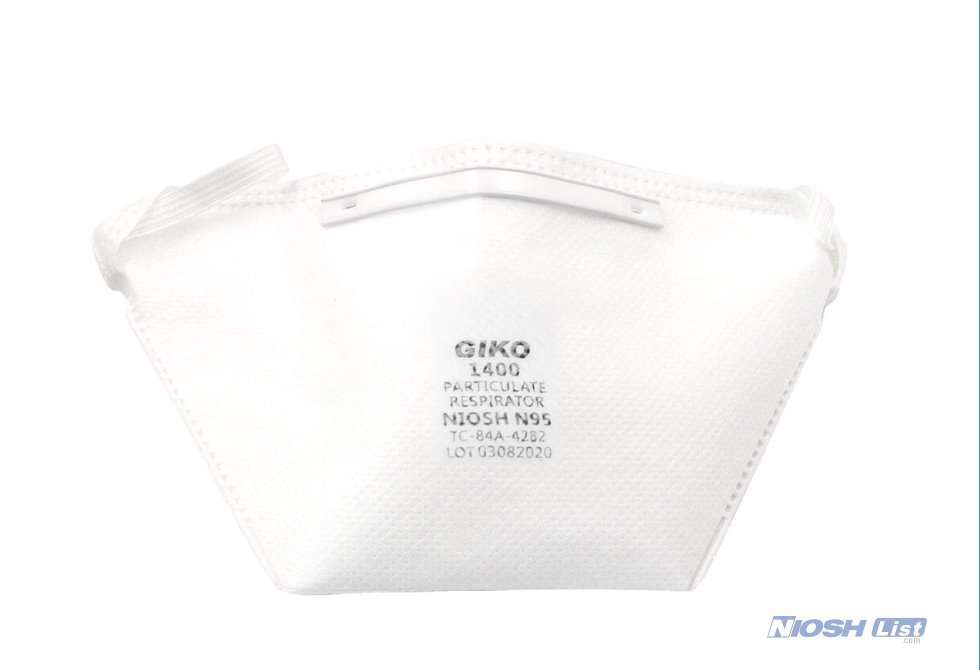
GIKO 1400 N95 mask for healthcare professionals with duckbill shape suits for most facial sizes with elastic headband and US average size.
Highly efficient filter media which is 95% efficient against a 0.3-micron particulate.
Duckbilled shape medical n95 mask is designed to increase comfort and wearability.
GIKO 1400 n95 respirators and surgical masks come with a white, elastic rubber headband and a closed-cell foam inside nosepiece, and a plastic-coated aluminum outside nosepiece.
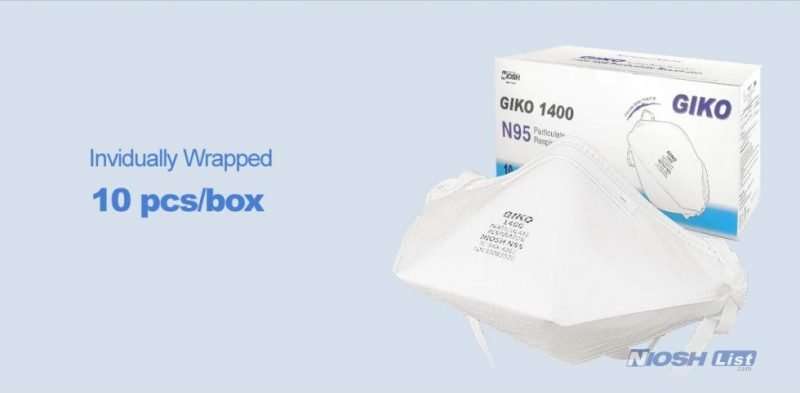
Individually wrapped for easy storage.
FDA cleared surgical mask.
ASTM Level 2 F2100 standard.
At least 95% filtration efficiency.
GIKO 1400 n95 surgical masks disposable medical grade FDA approved Certification
GIKO 1400 N95 NIOSH Approval Number TC-84A-4282, approval date 2/22/2006.

GIKO 1400 face mask surgical n95 Details
Size Medium
10pcs per box
Shelf life 2 Years