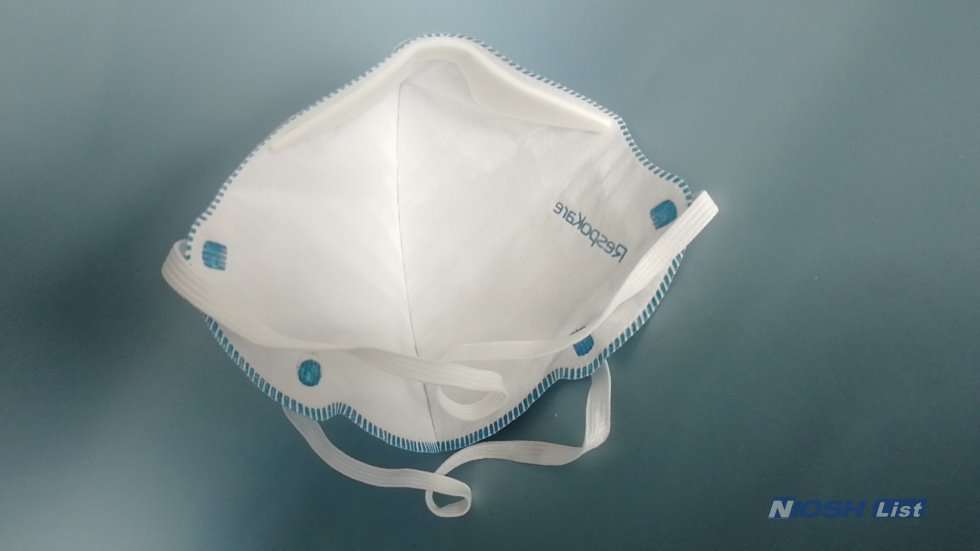
RespoKare N95 Surgical Respirator Mask With Antimicrobial/Antiviral Agent is FDA Cleared NIOSH Approved. Protect yourself from others against the spread of illness with the Flu Fighter Mask by RespoKare. The Respokare Niosh N95 Respirator Mask is the future of all masks, claiming to inactivate 99.9% of viral particles.

RespoKare RK200 3040A 3041A 3042A N95 Plus Respirator mask Overview
First and only FDA-Cleared Anti-Viral mask.viral particles.
NIOSH APPROVED (TC-84A-7796)
FDA 510K CLEARED: K122702
Sizing: Medium (M/S) best for slim/small faces (5.7 in from top of nose to chin). Size Large for regular adult faces (6.9 in from top of nose to chin)
U.S. patented 4-layer Innonix anti-viral technology – 10,000 times more effective than an ordinary mask
SARS and MERS – 99.99% inactivation of 15 different flu strains within 5 minutes
Measles + Airborne transmission – 99.99% inactivation of standard surrogate coronavirus within 1 minute
Tuberculosis – 88.97% inactivation within 10 minutes
Highly effective against 18 common Seasonal & Pandemic Influenza
RespoKare RK200 3040A 3041A 3042A N95 NIOSH Mask Certification
NIOSH Approved
Innonix RESPOKARE RK-200-3040A/3041A/3042A N95 NIOSH Approval Number TC-84A-7796, approval date 3/10/2017.
FDA cleared (510K)
Innonix RESPOKARE RK-200-3040A/3041A/3042A N95 mask passed FDA (510K) Cleared, worthy of consumer

Respokare RK200 3040A 3041A 3042A N95 Anti-Viral Protection Mask Details
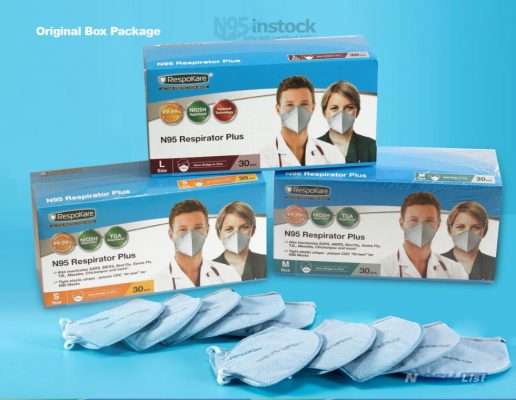
Size RK-200-3040A for small size. RK-200-3041A for medium size. RK-200-3042A for large size.
30pcs per box